validation of hardness tester slideshare|Hardness Tester Calibration & Certification Requirements : manufacturer The document discusses different hardness testing methods including Brinell hardness testing and Rockwell hardness testing. Brinell hardness testing involves pressing . webTop 10 Best Currency Exchange in Las Vegas, NV - February 2024 - Yelp - Foreign Money Exchange, Cashland, Xchange of America, Interchange, Moda Land Market Place, Travelex Currency Services, Death Valley Credit Union, Continental Currency of Nevada
{plog:ftitle_list}
webNo bairro históricamente mais importante do país reside um dos clubes mais tradicionais da cidade de São Paulo. Venha conhecer as instalações do Democrático do Ypiranga. .
SOP for Operation of Tablet Hardness Tester
charpy impact test brittle vs ductile
The document discusses different hardness testing methods including Brinell hardness testing and Rockwell hardness testing. Brinell hardness testing involves pressing .This document provides standard operating procedures for qualifying various .The document provides information about different hardness tests for materials, .The document discusses various hardness testing methods including indentation .
This document provides standard operating procedures for qualifying various laboratory equipment used in pharmaceutical quality control testing. It includes procedures for calibrating hardness testers, friability test .
The document provides information about different hardness tests for materials, including Brinell hardness test, Rockwell hardness test, and Vickers hardness test. It explains how each test is conducted, the equipment .Hardness Tester Calibration & Certification Requirements. Know the requirements of calibration of various hardness testers and successfully pass your next audit. There are many different .Indirect verification of hardness tester with reference blocks Hardness level/HRC Fig. 1. Example of a common pattern characterized by a rapid decrease at the higher hardness. .This document provides guidance for calibration and testing laboratories involved in hardness measurements, as well as their assessors. It has been produced to improve harmonization in .
Presentation Transcript. Objectives • To measure the hardness of the materials by Rockwell, Brinell and Vickers hardness test. • To become familiar with Combined Digital Hardness Tester. Introduction • Hardness is .
Quantitative Hardness Test. A small indenter is forced into the surface of a material to be tested, under controlled conditions of load and rate of application. The depth or size of the resulting . The document discusses various hardness testing methods including indentation hardness tests like Brinell, Vickers and Rockwell as well as microhardness tests. It provides details on the procedure, equipment, and .The principal purpose of the hardness test is to determine the suitability of a material for a given application, or the particular treatment to which the material has been subjected.
SOP for Calibration and Verification of Hardness Tester
4. Hugh M. Rockwell (1890–1957) and Stanley P. Rockwell (1886–1940) from Connecticut in the United States co-invented the "Rockwell hardness tester," a differential-depth machine. They applied for a patent on . 11. Hardness Testing: Indentation hardness • Brinell Non standard Hardness Test In this test, the loads are decreased and also the ball sizes are reduced. Ball indenters 1.25, 2.5 and 5.0 mm diameters with suitable . 12. 12 Compression is a critical step in the production of a tablet dosage form. The materials being compressed will need to have adequate flow and compression properties. The material should readily flow from the hopper .hardness, as shown in figure 1. -1.5-1.0-0.5 0.0 0.5 1.0 1.5 20 30 40 50 60 70 Indirect verification of hardness tester with reference blocks Hardness level/HRC Fig. 1. Example of a common pattern characterized by a rapid decrease at the higher hardness. Others common pattern are characterized by random
It defines validation and equipment qualification, which includes design qualification, installation qualification, operational qualification, and performance qualification. . is a deviation and must be corrected. A conclusion is drawn regarding the operation of equipment after the test functions are checked and all data has been analyzed .
11. Note: • As per official standard,tablet thickness variation alloted upto (+ or -) 5% of standard value. 4.HARDNESS TEST: • This test is also known as “Crushing Strength Test”. • Tablets require a certain amount of strength, or hardness to withstand mechanical shocks of handling in manufacture, packaging and shipping • Tablet hardness has been defined as the . Validation of capsules also involves validating the shell and contents along with encapsulation processes and testing. Process validation helps ensure quality and consistency of pharmaceutical products. . Microwave • Changing dryer techniques could affect such tablet properties such as hardness, disintegration ,dissolution & stability .
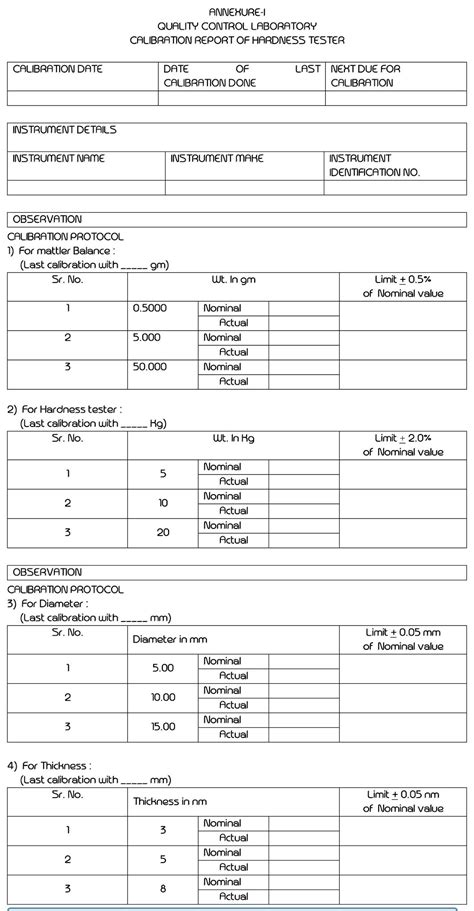
The work consists in the metrological characterization of the Primary Hardness Standard Machine (PHS Machine) realized by LTF S.p.A. for the Biroul Roman de Metrologie Legala (BRML), in Romania. Related: SOP for Cleaning of Tablet Hardness Tester 5.7 Calibration of Thickness 5.7.1 Select the menu item ‘Thickness’ from the sub menu ‘calibration’ and enter. 5. The hardness of the material is quantified using one of a variety of scales that directly or indirectly indicate the contact pressure involved in deforming the test surface. Since the indenter is pressed into the material during testing, hardness is also viewed as the ability of a material to resist compressive loads. Hardness tests are no longer limited to metals, and the . 25. Validation of Utility System This study provides a summary of the key validation test functions and acceptance criteria for each utility system. These are provided as a guideline for those involved in the validation of any pharmaceutical. Approval of this master plan neither provides approval of these test functions and acceptance criteria for does it limit the .
3. VALIDATION Validation is a tool of quality assurance which provides confirmation of the quality in equipment system, manufacturing process, software and testing methods. Validation of the individual step of manufacturing processes is called the process validation. WHY VALIDATION If would not be feasible to use equipment not knowing if it will .Calibration of Hardness Tester Learn how to verify the performance of Hardness Tester used to check the hardness of tablets. Ankur Choudhary 2024-04-17T15:59:53Z Print Online Courses Question Forum 2 comments
7. Mr.Balu.S.Khandare Quality assurance: Product quality cannot be assured for a process by routine quality control testing because of the limitation of statistical sampling. Validation changes the adequacy and reliability of a system or process to meet predetermined criteria. Economics: The direct economic benefit of validation is a reduction in the cost . validation of tablet and capsule formulation8 - Download as a PDF or view online for free . mixing/blending, granulation, drying, milling, lubrication, compression/filling, and testing. Process validation ensures consistent quality and safety of dosage forms. Read less. Read more . • An overdried granulation could result in poor hardness .The Vickers hardness testing method offers advantages such as a small indentation size, accurate hardness measurements, and applicability to a wide range of materials. Series measurements or mappings are very often carried out using this met-hod. However, it may not be suitable for very soft or highly textured materials, while alternative . 11. PROCESS VALIDATION OF SOLID DOSAGE FORMS(TABLET) A tablet is a most known solid pharmaceutical dosages form and comprises of a mixture of active substances and suitable excipients. .
The hardness tester resets automatically, tests the tablet samples with loop and can hold up to 100 samples printing results as a group. Model YD-2 fulfils USP <1217> on tablet orientation and 3-point calibration to ensure accuracy in tablet hardness testing. It comes with the calibration procedure as well. Calibration - Download as a PDF or view online for free. 5. Definitions "The measuring devices require calibration" Calibration in measurement technology and metrology is the comparison of measurement values delivered by a device under test with those of a calibration standard of known accuracy. Calibration is the process of adjusting an instrument . 9. The holes are equidistant from the centre of the plate and are equally spaced from one another. Attached to the under side of the lower plate is a woven stainless steel wire cloth with a plain square weave with 2.0 ±0.2 mm mesh apertures and with a wire diameter of 0.615 ±0.045 mm. The upper plate is covered with a stainless steel disc perforated by six . 5. Validation Protocol Process Validation of oral solid dosage form (Tablet)5 1. General information 2. Objective 3. Background/Prevalidation Activities Summary of development and tech transfer (from R&D or another site) activities to justify in-process testing and controls; any previous validations. 4.
9. • VALIDATION OF RAW MATERIALS AND EXCIPIENTS • validation of raw materials is one of the major causes of product variation or deviation from specification. The API may represent the most uncontrollable component in the complete product . The validation process of solid dosage form begins with the validation of raw materials ,both API and . The document describes research validating the hardness and tensile strength of Al 7075 hybrid composites using artificial neural networks. Experimental results for Al 7075 reinforced with fly ash and E-glass fibers were used to train a neural network model.
MAJOR STEPS IN THE DEVELOPMENT OF A VALIDATION PROGRAM From using test data determine the numerical range of each parameter : Eg tablet hardness of batches achieved an acceptable friability, disintegration, and dissolution. for a given parameter ; Establishing specification limits of extremes of acceptable hardness (high and low) provide . 11. • VALIDATION OF RAW MATERIALS AND EXCIPIENTS • validation of raw materials is one of the major causes of product variation or deviation from specification. The API may represent the most uncontrollable component in the complete product . The validation process of solid dosage form begins with the validation of raw materials ,both API and . Our Products • Complete range of Dissolution Testers • Dissolution Media Preparator • Disintegration Testers • Friability Testers • Tablet Hardness Testers • Electromagnetic Sieve Shakers • Tap Density Tester • Bulk Density Tester • Powder Flow Tester • Leak Tester • Peristaltic Pumps The information contained in this . 12. RIPER AUTONOMOUS NAAC & NBA (UG) SIRO- DSIR Raghavendra Institute of Pharmaceutical Education and Research - Autonomous K.R.Palli Cross, Chiyyedu, Anantapuramu, A. P- 515721 12 • This qualification is performed subsequent to installation and is repeated at certain intervals recommended by the manufacturer or defined by the customer. • .
Simplii Financial offers competitive interest rates on its produ.
validation of hardness tester slideshare|Hardness Tester Calibration & Certification Requirements